Que recherchez-vous ?
Un contenu sur ce site
Une personne sur l'annuaire
Une formation sur le catalogue
Un contenu sur ce site
Une personne sur l'annuaire
Une formation sur le catalogue
 Dans le cadre de la réorganisation imposée au laboratoire Signalisation et Transports Ioniques Membranaires (STIM), les équipes TIME et TIRC qui étaient dirigées par Frédéric Becq et Patrick Bois ont regroupé leurs activités au sein d’une seule et même unité de recherche. Cette unité, intitulée PRéTI pour « Physiopathologie et Régulation des Transports Ioniques », a pris son envol en tant qu’unité de recherche de l’université de Poitiers (UR 24 184) le 1er janvier 2022. Elle développe ses investigations autour des transports ioniques membranaires dans trois grands domaines : l’environnement cardiaque (axe dirigé par Aurélien Chatelier), le système musculaire squelettique (axe dirigé par Stéphane Sebille) et les membranes épithéliales (axe dirigé par Frédéric Becq).
Dans le cadre de la réorganisation imposée au laboratoire Signalisation et Transports Ioniques Membranaires (STIM), les équipes TIME et TIRC qui étaient dirigées par Frédéric Becq et Patrick Bois ont regroupé leurs activités au sein d’une seule et même unité de recherche. Cette unité, intitulée PRéTI pour « Physiopathologie et Régulation des Transports Ioniques », a pris son envol en tant qu’unité de recherche de l’université de Poitiers (UR 24 184) le 1er janvier 2022. Elle développe ses investigations autour des transports ioniques membranaires dans trois grands domaines : l’environnement cardiaque (axe dirigé par Aurélien Chatelier), le système musculaire squelettique (axe dirigé par Stéphane Sebille) et les membranes épithéliales (axe dirigé par Frédéric Becq).
Pour des raisons de lisibilité internationale, ces parties seront rédigées en anglais
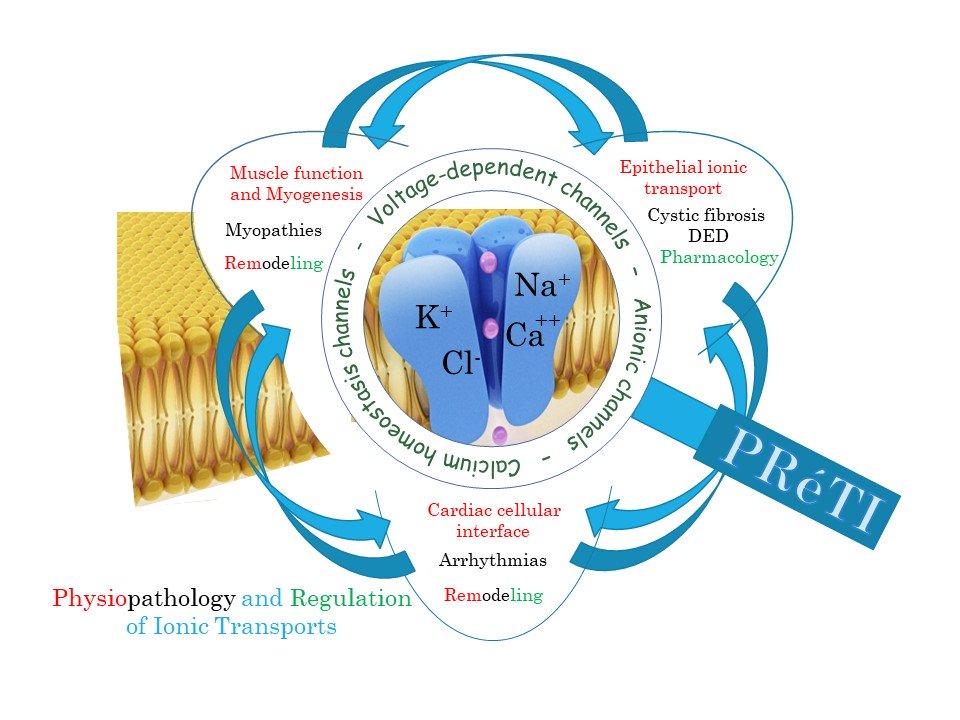
 Our main interest is focused on Cardiac tissue where the multiple cellular electrophysiological interfaces are crucial for integrative and coherent heart function and excitability. In this model, our research focuses on the characterization of new mechanisms involved in physiology and pathophysiology of cardiac muscle including their local innervation system. This concerns in particular the close relationship between the activity of ionic channels and calcium homeostasis at the cellular level, the electrophysiological and chemical interactions between the different cardiac cell types at the tissue level as well as their modulation and remodeling in pathological conditions. Our main objective here is to elucidate further the molecular causes responsible for cardiac dysfunctions and to identify new pharmacological targets.
Our main interest is focused on Cardiac tissue where the multiple cellular electrophysiological interfaces are crucial for integrative and coherent heart function and excitability. In this model, our research focuses on the characterization of new mechanisms involved in physiology and pathophysiology of cardiac muscle including their local innervation system. This concerns in particular the close relationship between the activity of ionic channels and calcium homeostasis at the cellular level, the electrophysiological and chemical interactions between the different cardiac cell types at the tissue level as well as their modulation and remodeling in pathological conditions. Our main objective here is to elucidate further the molecular causes responsible for cardiac dysfunctions and to identify new pharmacological targets.
 We are studying the physiological and pathological roles of chloride (Cl–) channels (especially the Cystic Fibrosis Transmembrane conductance Regulator chloride channel, CFTR) in human airway and ocular epithelial cells. Our cell models are epithelial cells of the airways and of the eyes with two human model diseases: Cystic Fibrosis (CF) and Dry-eye disease (DED). CF is the most common and best-known genetic disease involving a defect in transepithelial Cl–. It affects epithelial organs, i.e., lungs, pancreas, and intestine, among others. DED is a multifactorial malady of the tears and ocular surface that results in symptoms of discomfort, visual disturbances and tears film instability with potential damage to the ocular surface. Both are related to the regulation of transepithelial transport of ions and water.
We are studying the physiological and pathological roles of chloride (Cl–) channels (especially the Cystic Fibrosis Transmembrane conductance Regulator chloride channel, CFTR) in human airway and ocular epithelial cells. Our cell models are epithelial cells of the airways and of the eyes with two human model diseases: Cystic Fibrosis (CF) and Dry-eye disease (DED). CF is the most common and best-known genetic disease involving a defect in transepithelial Cl–. It affects epithelial organs, i.e., lungs, pancreas, and intestine, among others. DED is a multifactorial malady of the tears and ocular surface that results in symptoms of discomfort, visual disturbances and tears film instability with potential damage to the ocular surface. Both are related to the regulation of transepithelial transport of ions and water.
 Our main interest is focused on Musculoskeletal models where alterations of ion permeabilities and calcium homeostasis participate to myogenesis perturbation and muscular myopathies. Management of calcium transients definitely changes not only during the maturation of skeletal muscle cells, but also as muscular pathologies develop or as muscular tissue remodels in different conditions. Consequently, we are aiming at better understanding what are the relationships between calcium homeostasis and myogenesis, what is the interplay between calcium dysregulation and muscle diseases, especially in muscular dystrophy models such as human IPS-derived muscle cells, and how ion channels are involved in muscle remodelling under physiological conditions such as during exercise or under pathological conditions such as consecutive to obesity or hypertension.
Our main interest is focused on Musculoskeletal models where alterations of ion permeabilities and calcium homeostasis participate to myogenesis perturbation and muscular myopathies. Management of calcium transients definitely changes not only during the maturation of skeletal muscle cells, but also as muscular pathologies develop or as muscular tissue remodels in different conditions. Consequently, we are aiming at better understanding what are the relationships between calcium homeostasis and myogenesis, what is the interplay between calcium dysregulation and muscle diseases, especially in muscular dystrophy models such as human IPS-derived muscle cells, and how ion channels are involved in muscle remodelling under physiological conditions such as during exercise or under pathological conditions such as consecutive to obesity or hypertension.
 Transversal strategies merging together experimental approaches on the different models are a matter of major interest for PRéTI global development. Presently, actually striking activities of the laboratory relie 1) on the study of energy metabolism and 2) on the understanding of membrane lipid alterations observed in different pathological situations on the skeletal muscle, cardiac or pulmonary models.
Transversal strategies merging together experimental approaches on the different models are a matter of major interest for PRéTI global development. Presently, actually striking activities of the laboratory relie 1) on the study of energy metabolism and 2) on the understanding of membrane lipid alterations observed in different pathological situations on the skeletal muscle, cardiac or pulmonary models.